Fall 2022-Spring 2023 Semester Research

I worked on a project that involved preparing functionalized bilayer membranes and nanoplastics to investigate the influence of nanoplastics on biological systems via single molecule microscopy.
Institution
Georgia State University Department of Chemistry
Timeline
8 Months
Role
Undergraduate Student Researcher
Date Completed
May 2023
Summer 2022 Externship
Here is a synopsis of my research in the form of two images! Please continue to read below to learn more. You also have the option to watch me explain my research in three minutes. Check it out!
More About My Research Project:
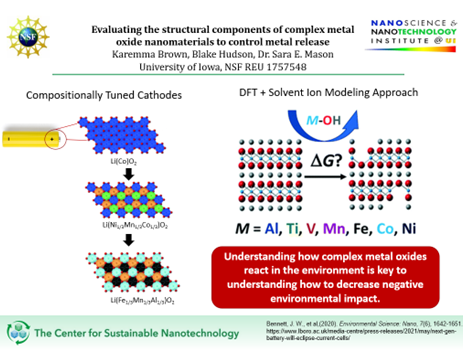
Title of Project: Evaluating the structural components of complex metal oxide nanomaterials to control metal release
Our cellphones are powered by batteries. These batteries are known as lithium-ion batteries. Like all batteries, they have a positive end and negative end. Our focus is on the positive end, called the cathode. If you were to break open the cathode, you would find clusters of nanomaterials called complex metal oxides. These complex metal oxides are crystalline solids that contain multiple metal cations and an oxide anion and are responsible for revolutionizing technology world-wide.
Without complex metal oxides, there would be no convenience of cellphones. Because you wouldn’t be able to recharge them! BUT there’s a hiccup, lack of recycling and improper disposal has introduced these complex metal oxides into the environment. This has had a negative environmental impact, the metal cations that make up complex metal oxides leak into the environment and are lethal to aquatic life. An example of observed release of toxic metal cations from a complex metal oxide is shown with a commercially successful battery material known as lithium nickel manganese cobalt oxide or NMC for short, which was the result of swapping transition metals of an older battery material called lithium cobalt oxide (LCO). While NMC is a higher performing and cheaper battery material than LCO, it is very toxic when not properly disposed as nickel and cobalt are released when exposed to water.
With this issue in mind, the goal of the Mason Lab is to understand what dictates cation release of complex metal oxides. To accomplish this, the Mason group first investigated the cation release of NMC by looking at the structure of NMC using a computational tool that you allows to model the structural components of these oxides using a computer, called the Density Functional Theory. The aim was to tailor cation release of these toxic metal cations under conditions where they would be less favorably released. What they discovered was by changing the amount of nickel and manganese, it allowed modification of cation release.
From that discovery, they decided next to swap the transition metals of lithium cobalt oxide with metals that formed more stable oxides and to compare their metal release to NMC, with the goal of finding the right recipe of metals in these oxides that would have low cation release. This summer, we continue this inquiry with the evaluation of the structural components of these complex metal oxides to control metal release, so that the next generation of battery materials will not have a negative environmental impact.
Company
The NSF Center for Sustainable Nanotechnology SURE Program
Timeline
2 Months
Role
Undergraduate Student Researcher
Date Completed
August 2022